The second set of hallmarks are the antagonistic ones. For most of our life, these processes are beneficial, but as we age they start to become damaging.
Deregulated nutrient sensing
When we eat, our body turns the food we consume into energy and nutrients. When we have nutrients, we can both increase our metabolism to make more energy, or use the nutrients for tasks that are needed for cell growth, like building proteins, putting together complex fat molecules, or building the A-C-G-T bases needed for DNA.
Whilst this is generally a good thing (if we didn’t have any nutrients and weren’t able to complete these tasks, we’d die pretty quickly) it can lead to problems over time. Our metabolism produces reactive oxygen species as a byproduct, and if the levels of these ROS build up then they can cause damage to other parts of the cell, including the DNA.
Likewise, we build components for DNA in order to have enough to copy it and make a new cell, either for growth of the tissue or to replace one that is too old. However, with every replication of DNA we run the risk of introducing more errors and mutations into the code, and also get closer to reaching the Hayflick limit where the telomeres run out and the the cell can no longer be reproduced. This topic was covered in the previous article, about the research of Judith Campisi.
When we have nutrients, it triggers pathways that result in building new proteins or tissues. These are called anabolic pathways (think of anabolic steroids contributing to building new muscle growth). If we increase these pathways too much, we end up doing damage to our cells more quickly, and thus decreasing longevity.
When we have fewer nutrients, our body instead follows catabolic pathways that slow down cell replication and break down the molecules we already have. In a very simple example, most diets work on the basis that when we don’t have food, we start breaking down the fat stores we already had). These pathways have been shown to increase longevity, and are one of the reasons why fasting has been shown to increase lifespan in mice (1). In humans, this can be mimicked with general caloric restriction, or newer studies suggest intermittent fasting could have the same effect (2,3). It has also led to studies linking the plant based, low red-meat, low saturated fat Mediterranean diet to longevity, as this diet doesn’t force the body through the wild high-low swings that an unhealthy western diet that is high in sugar and fat does (4,5).
It’s the difference between driving your car at 100mph for a short distance, or driving it at 30mph for a longer distance. If we want to live longer, we shouldn’t gorge ourselves on food.
Mitochondrial Dysfunction
Our mitochondria are the little pieces inside our cells that help us make energy from food. This process is called oxidative respiration, and turns glucose from broken down food into an energy source called ATP. However, as cells age, this process gets less and less efficient (6).
One of the reasons for this is that the mitochondria produce ROS (which were mentioned in the section above) when they work. ROS were always presumed to be the bad guys, a byproduct that showed our cells to be imperfect, causing damage that led to cancer, diabetes and aging (7–9). However, newer research shows that they have a much more nuanced role in our bodies, and that they are vital to things like the control of our immune system, and determining which cells need to die.
The balance of ROS being produced by a mitochondria is very tightly regulated. However, it can cause minor damage in the mitochondria. This can lead to mutations in the mtDNA, little loops of DNA that are within the mitochondria that just help make the things that the mitochondria specifically needs. It can also destabilise vital sections of the mitochondria, lead to fewer new mitochondria being made and the old damaged ones not being cleared properly. As time goes by, this damage accumulates. Once a mitochondria has reached a point of being too defective, it cannot regulate the ROS it produces, which leads to stress signals that in turn produce more ROS. All of this leads to more damage in the rest of the cell, which perpetuates the symptoms of aging more. Lots of skincare creams contain antioxidants which work to reduce the effect of ROS on the skin, and there are plans to repurpose old drugs to counteract ROS production in faulty mitochondria (10)
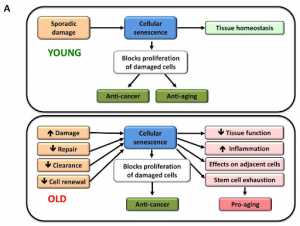
Cellular Senescence
This hallmark was covered in detail in a previous article of a scientific profile on Judith Campisi, whose work focuses on how cellular senescence links to aging. It is the point at which, for a multitude of reasons, our cells stop dividing .
Like the other hallmarks in this section, cellular senescence is an example of antagonistic pleiotropy, two very long words for quite a simple concept (11).
At the start of your life, cellular senescence is beneficial to your body, stopping damaged cells from being allowed to replicate themselves. However, once you’re old (this point differs from person to person, but physical and mental decline tends to start in your 50’s (12,13) the process stops being beneficial and instead becomes harmful, contributing to long term inflammation and cancer (14).
This is because the fundamental aim of evolution is to survive and reproduce. These genes that end up causing aging haven’t been removed from our DNA over time because they keep us alive and healthy until we are able to reproduce. It’s only once our best years are over that they start to be harmful. From this evolutionary perspective, the early benefits of these genes outweigh their later costs.
To tackle the downsides of cellular senescence, a class of drugs called Senolytics are being produced that will have the ability to selectively clear senescent cells from the body. However, this work is still in the very early stages (15). Additionally, the theory of antagonistic pleiotropy has been used to design a new method of treating cancer (16)
Figure 5A, from (17)
References
- Pifferi F, Terrien J, Marchal J, Dal-Pan A, Djelti F, Hardy I, et al. Caloric restriction increases lifespan but affects brain integrity in grey mouse lemur primates. Commun Biol. 2018 Apr 5;1:30.
- Hanjani NA, Vafa M. Protein Restriction, Epigenetic Diet, Intermittent Fasting as New Approaches for Preventing Age-associated Diseases. Int J Prev Med. 2018 Jun 29;9:58.
- Catterson JH, Khericha M, Dyson MC, Vincent AJ, Callard R, Haveron SM, et al. Short-Term, Intermittent Fasting Induces Long-Lasting Gut Health and TOR-Independent Lifespan Extension. Curr Biol. 2018 Jun 4;28(11):1714–24.e4.
- Melnik BC. Western diet-induced imbalances of FoxO1 and mTORC1 signalling promote the sebofollicular inflammasomopathy acne vulgaris. Exp Dermatol. Wiley; 2016 Feb;25(2):103–4.
- Capurso C, Bellanti F, Lo Buglio A, Vendemiale G. The Mediterranean Diet Slows Down the Progression of Aging and Helps to Prevent the Onset of Frailty: A Narrative Review. Nutrients [Internet]. 2019 Dec 21;12(1). Available from: http://dx.doi.org/10.3390/nu12010035
- Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011 Aug 26;333(6046):1109–12.
- Liou G-Y, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010 May;44(5):479–96.
- Kaneto H, Katakami N, Matsuhisa M, Matsuoka T-A. Role of Reactive Oxygen Species in the Progression of Type 2 Diabetes and Atherosclerosis. Mediators Inflamm [Internet]. 2010 Feb 16 [cited 2021 Aug 10];2010. Available from: https://www.hindawi.com/journals/mi/2010/453892/
- Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, et al. Oxidative stress, aging, and diseases. Clin Interv Aging. 2018 Apr 26;13:757–72.
- Detaille D, Pasdois P, Sémont A, Dos Santos P, Diolez P. An old medicine as a new drug to prevent mitochondrial complex I from producing oxygen radicals. PLoS One. 2019 May 2;14(5):e0216385.
- Williams GC. Pleiotropy, Natural Selection, and the Evolution of Senescence. Evolution. 1957;11(4):398–411.
- Salthouse TA. When does age-related cognitive decline begin? Neurobiol Aging. 2009 Apr;30(4):507–14.
- Milanović Z, Pantelić S, Trajković N, Sporiš G, Kostić R, James N. Age-related decrease in physical activity and functional fitness among elderly men and women. Clin Interv Aging. 2013 May 21;8:549–56.
- Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007 Sep;8(9):729–40.
- Kirkland JL, Tchkonia T. Senolytic drugs: from discovery to translation. J Intern Med. 2020 Nov;288(5):518–36.
- Lin KH, Rutter JC, Xie A, Pardieu B, Winn ET, Bello RD, et al. Using antagonistic pleiotropy to design a chemotherapy-induced evolutionary trap to target drug resistance in cancer. Nat Genet. 2020 Apr;52(4):408–17.
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013 Jun 6;153(6):1194–217.



Trackbacks/Pingbacks